HOUSTON — Doctors need 200 volunteers in Houston to take part in the final phase of a coronavirus vaccine clinical trial. The third phase will determine if the vaccine successfully reduces infections.
Several pharmaceutical companies are developing and testing vaccines to combat COVID-19.
UTHealth and Memorial Hermann are working together on the study involving AstraZeneca’s vaccine.
Over the next several weeks, 30,000 volunteers (including 200 in Houston) will be enrolled nationwide in the double-blind study. Researchers said 20,000 people will get two doses of the vaccine and 10,000 people will get two doses of a placebo.
Dr. Jessica Lee said the vaccine trial is historic. Lee is an assistant professor of internal medicine at McGovern Medical School at UTHealth.
“We’ve never done clinical trials in this United States with this kind of operation ... warp-speed on such a large and fast scale,” Lee said. “We’re trying to short cut some of the procedural paperwork, bureaucratic type of things. Not the safety.”
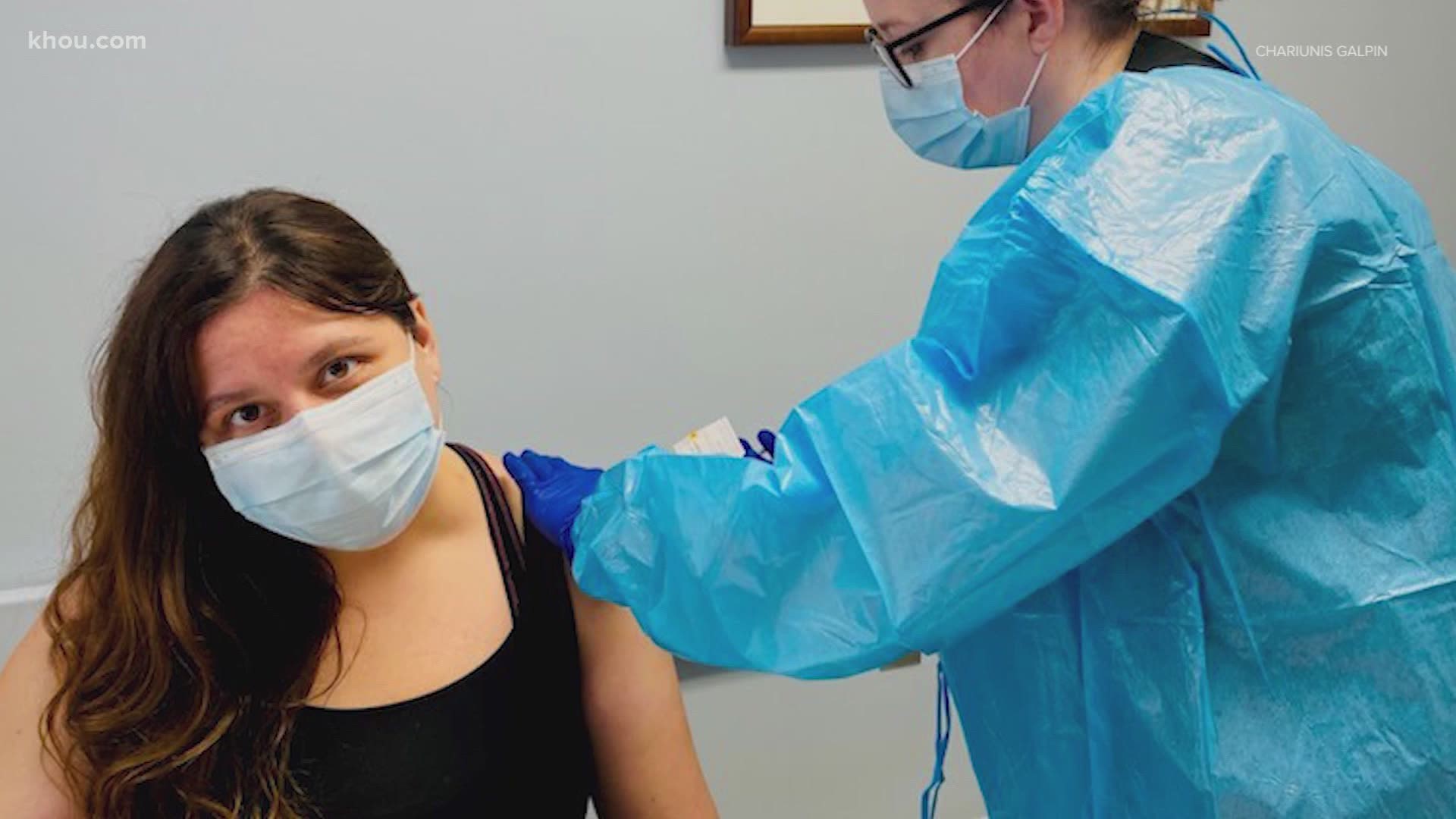
Participants in Houston started receiving the first dose of the vaccine on Friday. Chariunis and Matt Galpin said they did not hesitate to enroll in the trial.
“Obviously, we had to go through ... give more on our medical health history, but once we got down to the shot, it really is like a flu shot,” Chariunis Galpin said. “That’s one of the reasons why I did it. I just want to get back to being able to connect with people on a more normal level than what we’ve been doing right now.”
“I do feel a little safer, even though there’s a chance I got a placebo, of going out and interacting with people with my mask on,” Matt Galpin said.
He said his job puts him at a higher risk of getting the virus. Lee said they hope to enroll the 200 volunteers within eight weeks.
Participants must be 18 years or older, healthy or have medically stable chronic diseases, have no known history of SARS-CoV-2 infection, and be at an increased risk of infection.
“We also have a high emphasis on this particular trial on recruiting older patients as well as minorities. It’s really important to get these populations into this study. Because they’re so heavily affected by COVID, we really need them to participate in these trials,” Lee said.
The AstraZeneca clinical trial was on hold after three patients developed neurological side effects. Lee said the FDA just allowed the study to resume after determining the symptoms were not an effect of the vaccine, but rather the patients had a predisposition.
She said it's the final round of testing the COVID-19 vaccine before it is distributed to the general population.
“That’s part of the reason we want to get those 30,000 people so we can really see in a larger number of people the safety of this vaccine. I do believe it is going to be safe,” Lee said.
To volunteer, click here. For more information about the trial, click here or email COVID19vaccinestudy@uth.tmc.edu.